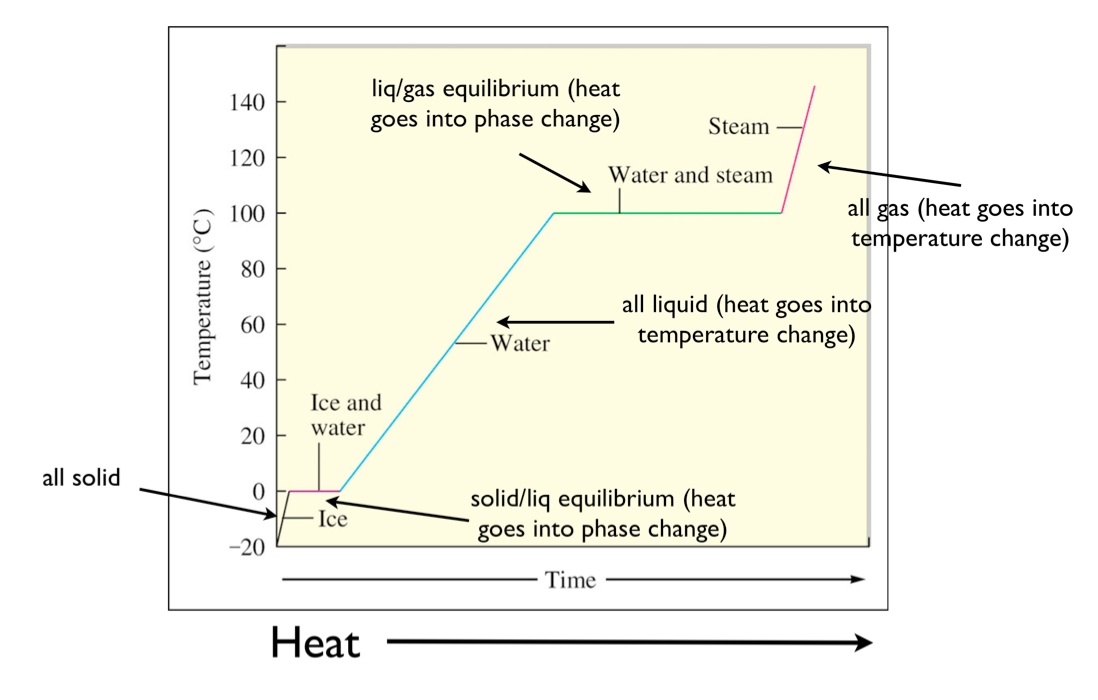
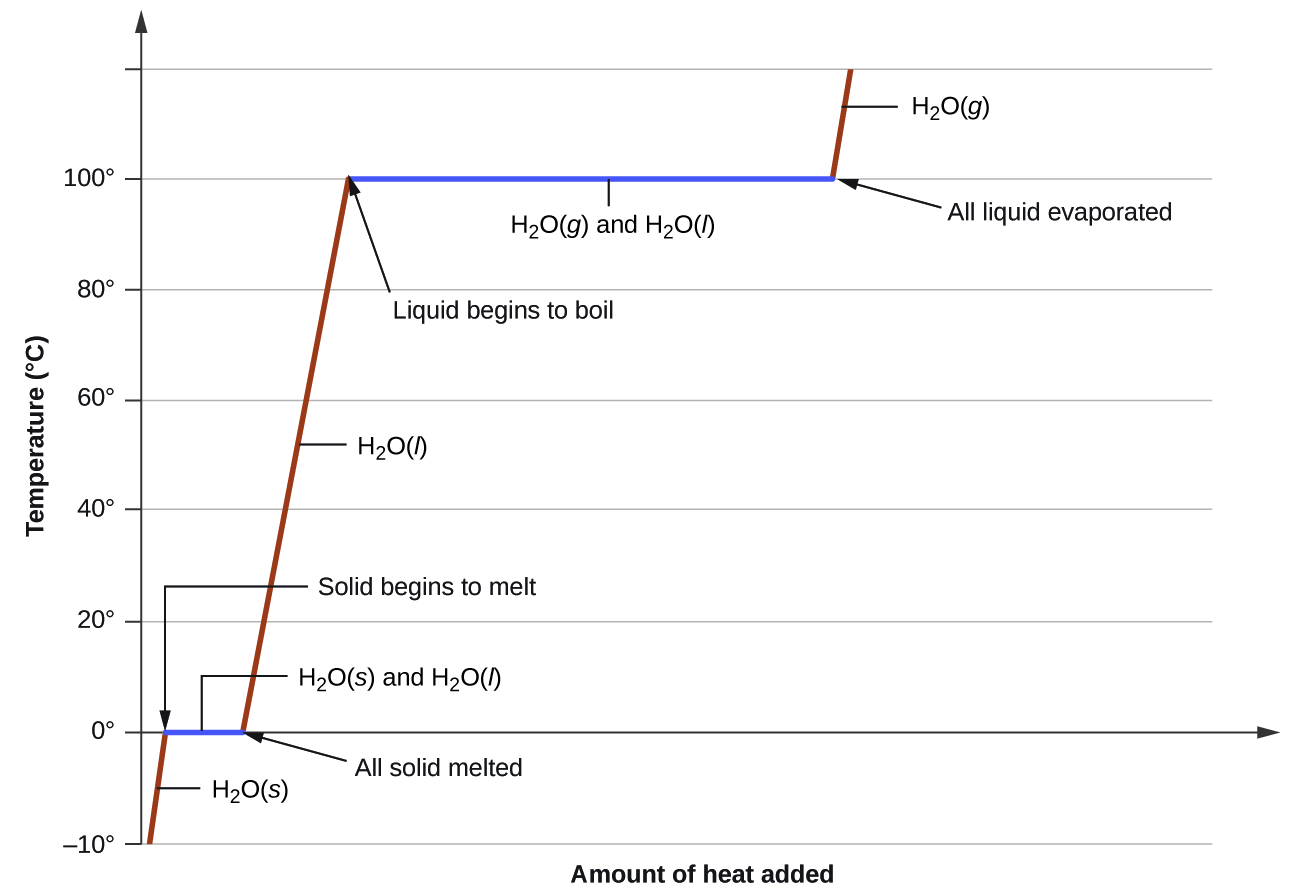
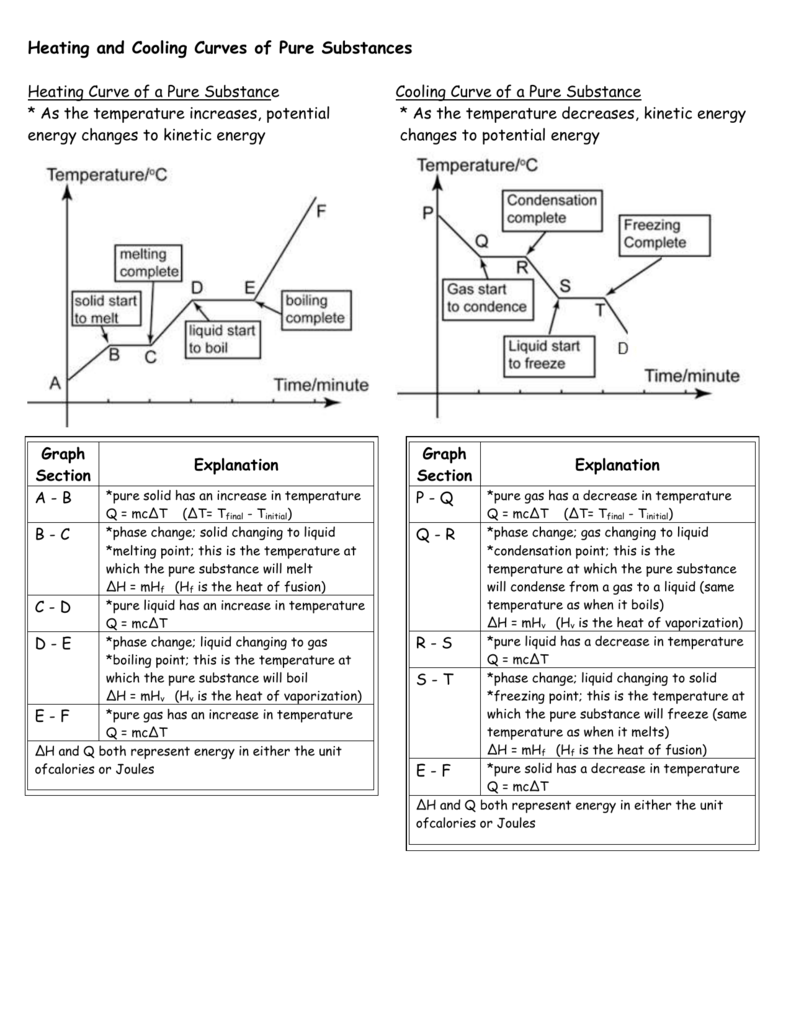
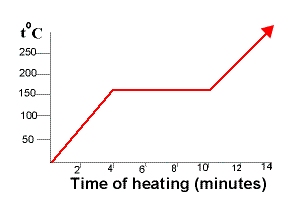
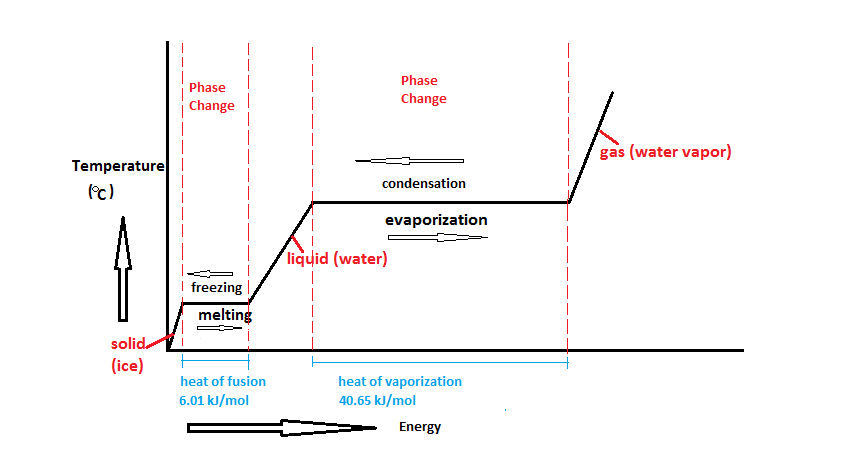
A typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat.
Cooling and heating curves of a pure substance.
The heating cooling curve of a pure substance so basically what we did today is the heating and cooling curve of a pure substance we also needed to do a flow chart because we might have a lab next class.
A b turns heat energy to kinetic energy.
Liquid solid and gas.
A heating curve shows how the temperature changes as a substance is heated up at a constant rate.
Figure pageindex 1 shows a typical heating curve.
In this video i will explain the concept of heating and cooling curves as they applies to water and ethanol.
A heating or cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing temperature.
B is the point where it starts to melt.
Temperature is plotted on the y axis while the x axis represents the heat that has been added.
Heat a substance and measure its temperature for.
As heat is steadily added to the ice block the water molecules will begin to vibrate faster and faster as they absorb kinetic energy.
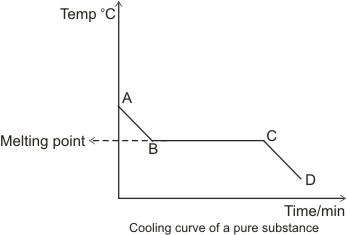
The graph shows the cooling curve for a sample of a compound called salol.
There are two possibilities.
They show how the temperature changes as a substance is cooled down.
Drawing a heating curve.
So a is a solid state at any temperature below melting point.
Cooling curves are the opposite.
Heating curves show how the temperature changes as a substance is heated up.
Plateaus in the curve regions of constant.
This class lesson was on the heating cooling curve of a pure substance.
Just like heating curves cooling curves have horizontal flat parts where the state changes from gas to liquid or from liquid to solid.
While a substance is undergoing a change in state its temperature remains constant.
A solid state at any temperature below its melting point particles packed closely together can only vibrate in a fixed position a b heat energy turns to kinetic energy when heated.
The temperature stays the same when a pure substance changes state the horizontal part of the graph shows that the salol.
Like many substances water can exist in different phases of matter.
The sloped areas of the graph represent a.